Abstract
Background: Chronic myeloid leukemia (CML) patients (pts) with the resistance at least to two tyrosine kinase inhibitors (TKIs) should be considered as candidates for allogeneic hematopoietic stem cell transplantation (allo-HSCT). At the same time, the majority of pts in real clinical practice continue conservative third-line therapy with TKIs (TKI-3L).
Aim: To compare the long-term results of TKI-3L vs allo-HSCT therapy in chronic phase (CP) CML.
Methods: We have included 139 pts in CP CML from six centers in Saint Petersburg and Leningrad Region (males, n=66) in the multicenter retrospective study: 73 pts on TKI-3L and 66 pts after allo-HSCT. Me age in the whole group was 45(19-88) mos. Basic characteristics of patients according to groups are presented in the Table. At the start of TKI-3L there were 51/73(71%) pts with no cytogenetic response (CyR). The best baseline CyR was minimal or minor (mmCyR), partial (PCyR) in 12/73(16%), 10/73(13%) cases, respectively. In the group with allo-HSCT there were 62/66(93%) pts with no CyR. The best baseline CyR was PCyR in 3(5%) cases. Reasons for TKI-2L discontinuation were: resistance in 56/73(76%) or intolerance in 17/73(24%) cases respectively. Reasons for performing allo-HSCT were: resistance in 65/66(98%), including 6 interferon-resistant cases, or intolerance in 1/66(2%) cases respectively. Descriptive statistics were used for analysis, significance of differences between groups were estimated using chi-square test and Fisher's exact test. The rate of complete CyR (CCyR) (equal to BCR::ABL transcript level ≤1% by IS) were assessed. Overall (OS) and progression-free survival (PFS) were analyzed by Kaplan-Meier method. Log-rank test was used to assess the significance of differences in survival between patients' groups.
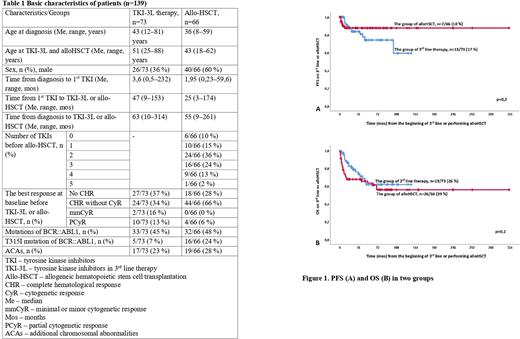
Results: The Me duration of TKI-3L therapy was 14(1-120) mos, the Me time of follow-up (FU) from initiation of TKI-3L was 25(3-136) mos. The Me duration time of FU from allo-HSCT was 42(1-322) mos. CCyR and MMR were achieved in 22/73(30%) and 9/73(12%) pts respectively in TKI-3L group while CCyR and PCR-negativity were achieved in 53/66(80%) and 29/66(44%) pts respectively in the group after allo-HSCT (with p≤0,05 in CCyR and MR in both groups). Progression to acceleration phase or blast crisis (AP/BC) was observed in 13/73(17%) pts with the Me time to progression 17 mos in TKI-3L group and in 7/66(10%) pts with the Me time to progression 6 mos in allo-HSCT group, p=0,24 (See Fig. 1A). Death occurred in 19/73(26%), including five deaths from non-CML-related causes with Me time to death 17,2 mos in TKI-3L group and in 26/66(39%), including 17 transplant-related deaths and 6 CML-related with Me time to death 14 mos in allo-HSCT group, p=0,25 (See Fig. 1B). Estimated 1-year, 3-year and 5-year OS was 95%, 65% and 65% respectively in TKI-3L group as well as 90%, 65% and 60% respectively in allo-HSCT group. During FU time Me PFS and OS was not reached in both groups. In pts with any CyR at baseline in TKI-3L group with FU time 25(3-136) mos no pts progressed to AP/BC and only 2 CML-unrelated deaths occurred. At the last visit, TKI-3L therapy was continued in 13/73(18%) with Me FU time 7,5(4-99) mos. Most of them(n=10) with CCyR or better responses. TKI-3L discontinuation was mainly associated with resistance in 42/60(70%) pts. In allo-HSCT group 40/66(61%) pts alive with 21/40(53%) in stable PCR-negativity.
Conclusion: In our observation OS and PFS was equal under TKI-3L or after allo-HSCT. No Pts with any CyR at baseline progressed and dead due to CML in TKI-3L group. Meanwhile less than one third pts and a few of them obtained CCyR and MMR, respectively, whereas the majority of pts after allo-HSCT reach CCyR and nearly half of them obtained PCR-negativity in this heavily pretreated group. Despite there are some biases (retrospective study, undirected comparison, in allo-HSCT group were included pts with any number TKIs or even without ones, much less FU time in the TKI-3L group), it seems that allo-HSCT is preferable option for pt after two second generation TKIs failure. Also in pts with any CyR after 2TKIs failure the use of TKI-3L therapy might be justified.
Disclosures
Lomaia:Fusion Pharma: Speakers Bureau; Bristol Myers Squibb: Other: Travel, Accommodation, Expenses ; Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau. Motorin:Novartis: Speakers Bureau; Pfizer: Speakers Bureau. Voloshin:Pfizer: Speakers Bureau. Efremova:Novartis: Honoraria, Speakers Bureau. Alexeeva:AbbVie: Speakers Bureau. Martynkevich:Novartis: Speakers Bureau; Pfizer: Speakers Bureau. Medvedeva:Janssen: Speakers Bureau; Soteks: Speakers Bureau; Sanofi-Aventis: Speakers Bureau. Shuvaev:Novartis: Other: Expert opinion, Speakers Bureau; Pfizer: Speakers Bureau; Amgen: Speakers Bureau; AbbVie: Speakers Bureau. Morozova:Novartis: Speakers Bureau; AMGen: Speakers Bureau; Pfizer: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.